Gospel of patients with hip fractures, new drugs can effectively enhance physical fitness
November 29, 2017 Source: WuXi PharmaTech
Window._bd_share_config={ "common":{ "bdSnsKey":{ },"bdText":"","bdMini":"2","bdMiniList":false,"bdPic":"","bdStyle":" 0","bdSize":"16"},"share":{ }};with(document)0[(getElementsByTagName('head')[0]||body).appendChild(createElement('script')) .src='http://bdimg.share.baidu.com/static/api/js/share.js?v=89860593.js?cdnversion='+~(-new Date()/36e5)];Viking Therapeutics today announced the positive top line results of the VK5211. The trial conducted a 12-week Phase 2 clinical study of patients with hip fractures. The data showed that the trial reached the primary endpoint, and that dose-dependent VK5211 significantly increased lean body mass compared to placebo. The study also reached other important secondary endpoints. In addition, VK5211 showed good safety and tolerability in this study, and there were no drug-related serious adverse events (SAE) reports.

A hip fracture is one of the most serious injuries caused by a fall. Patients with hip fractures are difficult to recover and many people cannot live alone. In the United States, more than 300,000 elderly people aged 65 years and older are hospitalized each year for hip fractures, and more than 95% of hip fractures are caused by falls, most of which are caused by side falls. Women often suffer from osteoporosis, a disease that weakens bones and makes bones more likely to break. Women account for three-quarters of hip fractures. As the population ages, the number of hip fractures may continue to increase.
VK5211 is an oral non-steroidal selective androgen receptor modulator (SARM) for the treatment of patients recovering from non-selective hip fracture surgery. Due to the tissue selective mechanism of action and the oral route of administration, VK5211 may produce testosterone with therapeutic benefit, improving safety, tolerability and patient acceptance. In the phase 1 clinical trial, the lean body mass increased significantly after 21 days of VK5211 treatment. In the preclinical model of osteoporosis, VK5211 showed an improvement in bone mineral density and content, bone strength and other indicators.
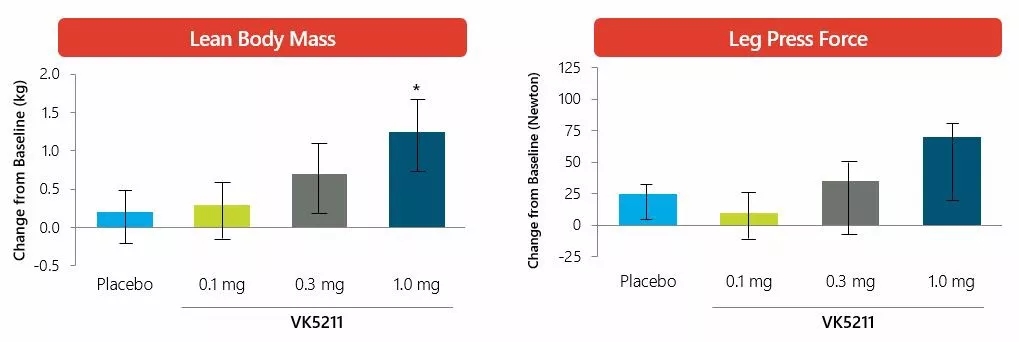
â–² VK5211 has obtained good experimental data in Phase 1 clinical trial (Source: Viking Therapeutics official website)
This Phase 2 clinical trial was a randomized, double-blind, placebo-controlled, parallel-group international study designed to assess the efficacy, safety, and tolerability of VK5211 in patients recovering from hip fracture surgery. A total of 108 patients were randomized to receive VK5211 (0.5 mg, 1.0 mg, 2.0 mg) once daily or placebo for 12 weeks.
Studies have shown that all doses of VK5211 have reached the primary end point, with statistically significant increase in total lean body mass (less head). Placebo-adjusted lean body mass increased by 4.8% (0.5 mg, p < 0.005), 7.2% (1.0 mg, p < 0.001), and 9.1% (2.0 mg, p < 0.001), respectively, an increase of 1.6 kg. , 2.5 kg and 3.1 kg lean body mass. The proportion of patients with at least a 5% increase in total lean body mass was 19% (placebo), 61% (0.5 mg), 65% (1.0 mg), and 75% (2.0 mg), with an increase of 2.0 kg. 14% (placebo), 57% (0.5 mg), 65% (1.0 mg), and 81% (2.0 mg), respectively.
All doses of VK5211 also reached a secondary efficacy endpoint. Placebo adjusted lean body mass increased by 6.1% (0.5 mg, p < 0.01), 9.0% (1.0 mg, p < 0.001) and 10.2% (2.0 mg, p < 0.001), respectively. This is equivalent to an increase of 0.8 kg, 1.3 kg and 1.4 kg respectively. The placebo-adjusted total body mass gain increased by 4.7% (0.5 mg, p < 0.005), 6.8% (0.5 mg, p < 0.001), 8.3% (2.0 mg, p < 0.001). This is equivalent to an increase of 1.7 kg, 2.6 kg and 3.1 kg respectively.
â–² VK5211 is expected to improve the lives of patients in many ways (Source: Viking Therapeutics official website)
“We are very pleased that this trial has been successful in the primary efficacy endpoint and important secondary endpoints, demonstrating the potential benefits of VK5211 in this case, the study was statistically significant at all doses, and there were clear doses Dependence provides evidence of an efficacious pharmacological effect of VK5211 on muscle growth,†Dr. Brian Lian, CEO of Viking, said: “We are also encouraged by the initial safety and tolerability characteristics of VK5211, especially since there is no It is important to observe drug-related SAE, because hip fracture patients are very fragile, and many of them with multiple diseases require multiple treatments. We are happy to see VK5211 produced in this challenging population. Such a powerful efficacy and safety."
Dr. Jay Magaziner, professor and head of the Department of Epidemiology and Public Health at the University of Maryland School of Medicine, said that the quality of the lean body in the elderly who are prone to lose muscle is impressive. Such a drug that can be easily taken orally can provide valuable aids for other treatment strategies, such as physical therapy and protein-rich diets, after hip fractures, increasing the chances of patients returning to normal activities."
We congratulate this new drug for its positive phase 2 clinical results, and we look forward to continuing the smooth progress in the next trial to bring rehabilitation to more elderly patients with fractures.
Reference materials:
[1] Viking Therapeutics Announces Positive Top-Line Results From Phase II Study of VK5211 in Patients Recovering From Hip Fracture
[2] Viking Therapeutics official website
Powder Fire Extinguisher,Powder Type Fire Extinguisher,Dry Powder Fire Extinguisher,Dry Powder Extinguisher
JIANGSU NEW FIRE FIGHTING TECHNOLOGY CO.,LTD , https://www.newayfire.com